Abstract
In recent years, stress levels have increased considerably following the COVID-19 pandemic and socioeconomic climate. This brief systematic review (SR) article aims to integrate the findings of several clinical trials to determine the efficacy of Ashwagandha Dietary Supplement (ADS) for perceived stress and anxiety management. This meta-analysis (MA) emphasizes the Perceived Stress Scale (PSS) and the Hamilton Anxiety Rating Scale (HAM-A) scoring systems. The results of the MA show that ashwagandha extract significantly reduced stress (PSS: Overall effect size, Cohen's d: −1.34; 95% CI: −1.86 to −0.81; I2 = 83%, P = 0.00) and anxiety (HAM-A: Overall effect size, Cohen's d: −1.96; 95% CI: −3.64 to −0.27; I2 = 94%, P = 0.02) compared with placebo/control. Based on this information, the authors believe that Ashwagandha can be a useful supplement to help reduce perceived stress and anxiety. Additionally, future research with increased sample sizes will provide more insights into the precise dosage and duration needed for optimal stress reduction efficacy. Keywords: Ashwagandha, Dietary Supplement, Withaferin A, Stress Relief, Meta-Analysis.
Introduction
The American Psychological Association conducted a study in 2023 that found that the long-term effects of stress brought on by the COVID-19 pandemic and socio-economic climate in recent years have led to an increase in chronic health conditions nationwide. In this study, 81% of people reported that their mental health was good or better, but 37% reported being diagnosed with a mental health condition. This increased by 5% from the pre-pandemic reports.[1] Stress plays a substantial role in every aspect of human life, including social, personal, and professional elements. Therefore, stress exerts a fundamental impact on overall well-being and productivity. Evidence suggests that the use of herbal and plant preparations known as adaptogens can help the body tolerate, resist, and adapt to stress, thus substantially decreasing stress levels. Ashwagandha belongs to this group of herbal substances that aid the body in accommodating stressors.[2] Ashwagandha has garnered attention for its stress-reducing properties and is supported by extensive research.[3] Incorporating natural supplements like Ashwagandha could be a valuable strategy for mitigating the effects of stress and promoting overall wellness.
Ashwagandha, also known as Withania Somnifera, comes from a perennial shrub native to many parts of the world, particularly South Asia and Africa. Ashwagandha contains a group of compounds called withanolides which are known to have anti-inflammatory and antioxidant properties.[4] These compounds also have GABAergic [5] and serotonergic activity [6] which modulate brain neurotransmitters, leading to reduced anxiety and depression, thereby also reducing stress. Withaferin A, the most prevalent withanolide in Ashwagandha has an oral bioavailability of 1.8%.[7] Extracts can be sourced from both the leaves and roots of a mature plant, however, the presence of withanolides is more prevalent in the roots than any other part of the plant.[8]
Numerous clinical trials have indicated that Ashwagandha may help in reducing stress; however, the results are somewhat mixed. One article found that the Ashwagandha treatment group did not show a significantly greater impact on stress reduction than the placebo group. [9] Ashwagandha supplements are currently available in the U.S. in powder, capsule, tablet, and gummy dosage forms. The regulation of dietary supplements falls under the Dietary Supplement Health and Education Act of 1994 (DSHEA) and states that manufacturers of dietary supplements are prohibited from producing misbranded or adulterated products and are responsible for evaluating the safety and labeling of their products before marketing.[10] Therefore, it is pivotal that any consumer of dietary supplements verifies sources and quality control practices implemented by supplement manufacturers.[10] The objective of this review is to determine the efficacy of Ashwagandha supplementation in stress reduction by consolidating the results of several clinical trials that implemented either the PSS or HAM-A scale.
Method
A systematic search was conducted for the effects of Ashwagandha on stress reduction using multiple online journal databases, including PubMed and Google Scholar. This search focused on randomized controlled clinical trials. Participant demographics and methods used to measure the efficacy of Ashwagandha vary between studies.
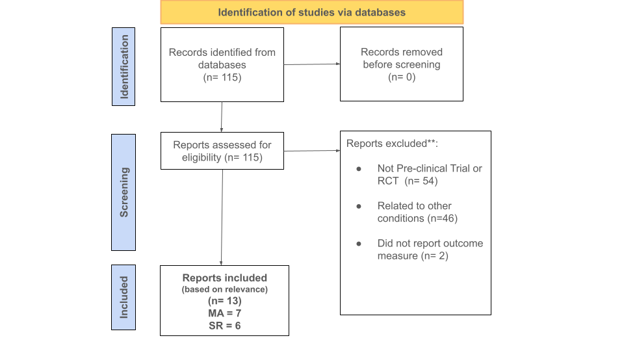
Figure 1: Flow Chart and Study Selection showing the number of articles identified after the initial keyword search and the number of articles included after the final screening.
Results and Discussion
In this SR and MA, thirteen clinical trials were regarded. Six of these were used to conduct the MA while seven more were included in the SR. Several factors varied among these clinical trials, including participant demographics, trial duration, supplement dosages, and outcomes. The dosages included in these clinical trials ranged from 125 mg to 600 mg with durations of 56 days to 90 days. Most of the clinical trials included in this MA were conducted within 60 days, so for comparison, data was extracted from this time point. Only one article out of the six included in this MA reported no significant effect, while all of the other studies reported a significant effect of Ashwagandha for stress reduction. [9] Although this article reported no significant effect, an improvement in stress reduction was reported, still suggesting that ashwagandha may have a positive impact on stress management as a supplement. These outcomes will be further discussed in subsequent sections to provide a comprehensive analysis of the impact of Ashwagandha on mental stress relief.
Table 1: Study Designs and Outcomes of the Clinical Trials.
| Author (Year) | Dose | Duration | Participants | % weight | Outcomes |
| Chandrasekhar (2012) [11] | 600 mg/day | 60 days | 61 | 11 | A statistically significant reduction in PSS scores was reported. |
| Lopresti (2019) [12] | 240 mg/day | 60 days | 60 | 10 | A statistically significant reduction in HAM-A scores was reported. |
| Salve (2019) [13] | 600 mg/day | 56 days | 58 | 10 | A significant reduction in PSS scores and HAM-A scores was observed. |
| Gopukumar (2021) [14] | 300 mg/day | 90 days | 125 | 22 | A statistically significant reduction in PSS score was observed. |
| Majeed (2023) [15] | 500 mg/day | 60 days | 54 | 9 | A statistically significant reduction in PSS score was observed. |
| Smith (2023) [9] | 400 mg/day | 84 days | 120 | 21 | A reduction in PSS score was observed, however, the improvements were not significantly different from participants taking a placebo |
| Pandit (2024) [16] | 500 mg/day | 56 days | 98 | 17 | A significant reduction in PSS scores and HAM-A scores was observed. |
The variability of diagnostic tools among clinical trials compelled the decision to employ two different methods, the PSS (Perceived Stress Score) and HAM-A scale (Hamilton Anxiety Rating scale), in this MA. [16] These were the two most commonly utilized methods for evaluating the effects of Ashwagandha on stress management. Among the articles reviewed for this MA, six clinical trials implemented the PSS and three implemented the HAM-A scale. This MA aims to assess the effectiveness of Ashwagandha for stress reduction by evaluating the outcomes of both diagnostic methods.
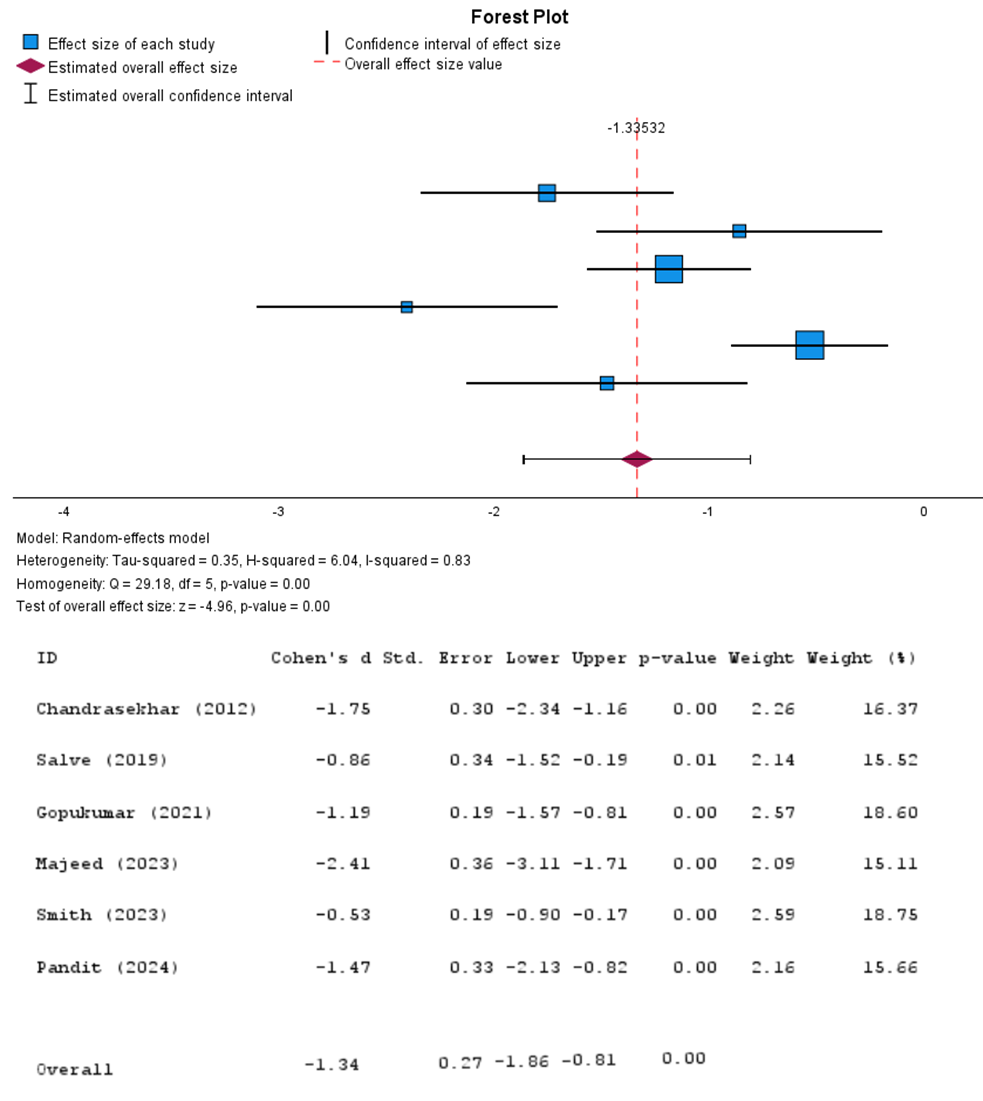
Figure 2: Forest plot (Ashwagandha vs. Control): MA on PSS score (Random-effects model)
Data from six clinical trials conducted between 2012 to 2024 were included in the analysis for the PSS method. Cohen’s d is often used to differentiate between the sizes of two groups, allowing for the determination of effectiveness regarding treatment vs placebo. In this study, Cohen’s d, which indicated the overall effect size, was calculated to be -1.34 (95% CI: -1.86 to -0.81), suggesting a significant reduction in perceived stress levels among participants taking Ashwagandha. The P-value for the analysis was reported to be 0.00, which indicates a statistically significant effect. The heterogeneity analysis revealed an I2 value of 83%, suggesting moderate to high heterogeneity among the studies included in the MA. This can be explained by the distinct differences in variables across studies including duration, dosage strengths, and participant demographics.
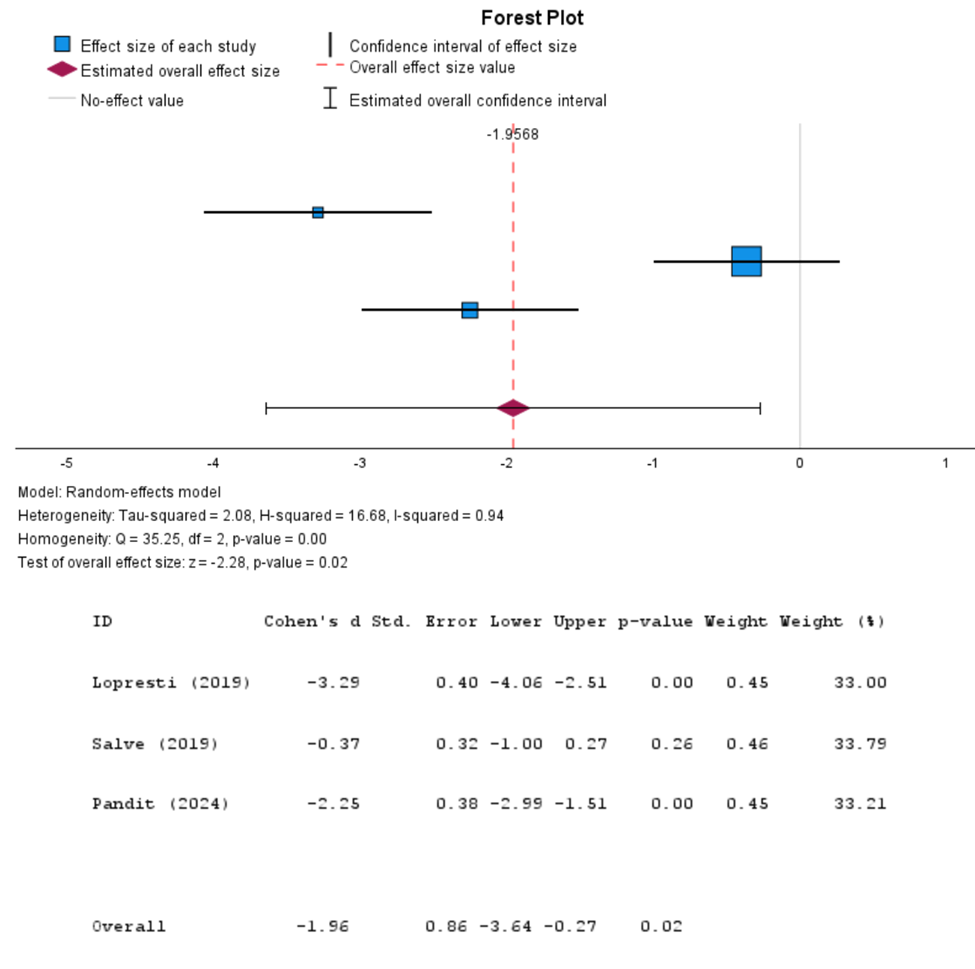
Figure 3: Forest plot (Ashwagandha vs. Control): a MA on HAM-A score (Random-effects model)
For the HAM-A method, Cohen’s d was calculated to be -1.96 (95% CI: -3.64 to -0.27, P-value: 0.02), suggesting a significant reduction in perceived stress levels among participants taking Ashwagandha. The heterogeneity analysis revealed an I2 value of 94%, suggesting high heterogeneity among the studies included in the MA. After comparing our MA with the findings of several other studies (Standardized Mean Difference: −1.75; 95% CI: −2.29, −1.22; p = .005; I2 = 83.1%) [17], (Mean Difference = −6.01, 96%CI: −18,20 6,19, p = 0.168) [18] it can be determined that our findings are similar to the results of the others. The consistency among our findings and the results identified by others further validates the likelihood that Ashwagandha is efficacious at reducing stress MA, none of the participants reported any serious adverse events, suggesting that Ashwagandha is safe for consumption as a dietary supplement. [19]
Limitations of this Study
A significant limitation of this MA is the heterogeneity (I2 > 0.8) due to variations in study size, participant demographics, diagnostic methods, duration, and dosage strength. Therefore, the validity of the results in this study may be compromised.
Conclusion
The results of this MA correspond with the results of other studies, thereby indicating the efficacy of Ashwagandha for stress reduction. Furthermore, the effect size, confidence interval, and p-values produced by this study further support the evidence that Ashwagandha improves mental stress management. However, the moderate heterogeneity of the PSS method and the high heterogeneity of the HAM-A method provide a basis to use caution when interpreting these results. Henceforth, consumers must validate the authenticity and quality assurance methods implemented by supplement manufacturers. Although no severe adverse effects were reported in the clinical trials included in this MA, future studies involving larger sample sizes and uniform diagnostic methods are essential to comprehensively cultivate the therapeutic efficacy and safety profile of Ashwagandha for stress reduction.
Conflict of interest
The authors declare no conflict of interest.
References
- Stress in America 2023: A nation recovering from collective trauma. https://www.apa.org. Accessed September 16, 2024. https://www.apa.org/news/press/releases/stress/2023/collective-trauma-recovery
- What are adaptogens and should you be taking them? Accessed September 16, 2024. https://www.uclahealth.org/news/article/what-are-adaptogens-and-should-you-be-taking-them
- Provino R. The Role of Adaptogens in Stress Management. Aust J Med Herbal. 2020;22(2):41-49. doi:10.3316/informit.141015308096078
- Ashwagandha: Usefulness and Safety. NCCIH. Accessed September 16, 2024. https://www.nccih.nih.gov/health/ashwagandha
- Nuss P. Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr Dis Treat. 2015;11:165-175. doi:10.2147/NDT.S58841
- Speers AB, Cabey KA, Soumyanath A, Wright KM. Effects of Withania somnifera (Ashwagandha) on Stress and the Stress-Related Neuropsychiatric Disorders Anxiety, Depression, and Insomnia. Curr Neuropharmacol. 2021;19(9):1468-1495. doi:10.2174/1570159X19666210712151556
- Gupta SK, Jadhav S, Gohil D, et al. Safety, toxicity and pharmacokinetic assessment of oral Withaferin-A in mice. Toxicol Rep. 2022;9:1204-1212. doi:10.1016/j.toxrep.2022.05.012
- Mir BA, Khazir J, Hakeem KR, Kumar A, Koul S. Withanolides array of Withania ashwagandha sp. novo populations from India. Ind Crops Prod. 2014;59:9-13. doi:10.1016/j.indcrop.2014.04.024
- Smith SJ, Lopresti AL, Fairchild TJ. Exploring the efficacy and safety of a novel standardized ashwagandha (Withania somnifera) root extract (Witholytin®) in adults experiencing high stress and fatigue in a randomized, double-blind, placebo-controlled trial. J Psychopharmacol Oxf Engl. 2023;37(11):1091-1104. doi:10.1177/02698811231200023
- Program HF. Dietary Supplements. FDA. August 29, 2024. Accessed September 16, 2024. https://www.fda.gov/food/dietary-supplements
- A Prospective, Randomized Double-Blind, Placebo-Controlled Study of Safety and Efficacy of a High-Concentration Full-Spectrum Extract of Ashwagandha Root in Reducing Stress and Anxiety in Adults. Accessed September 17, 2024. https://journals.sagepub.com/doi/epdf/10.4103/0253-7176.106022
- Lopresti AL, Smith SJ, Malvi H, Kodgule R. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract. Medicine (Baltimore). 2019;98(37):e17186. doi:10.1097/MD.0000000000017186
- Salve J, Pate S, Debnath K, Langade D. Adaptogenic and Anxiolytic Effects of Ashwagandha Root Extract in Healthy Adults: A Double-blind, Randomized, Placebo-controlled Clinical Study. Cureus. 11(12):e6466. doi:10.7759/cureus.6466
- Gopukumar K, Thanawala S, Somepalli V, Rao TSS, Thamatam VB, Chauhan S. Efficacy and Safety of Ashwagandha Root Extract on Cognitive Functions in Healthy, Stressed Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Evid Based Complement Alternat Med. 2021;2021(1):8254344. doi:10.1155/2021/8254344
- Majeed M, Nagabhushanam K, Mundkur L. A standardized Ashwagandha root extract alleviates stress, anxiety, and improves quality of life in healthy adults by modulating stress hormones: Results from a randomized, double-blind, placebo-controlled study. Medicine (Baltimore). 2023;102(41):e35521. doi:10.1097/MD.0000000000035521
- Pandit S, Srivastav AK, Sur TK, Chaudhuri S, Wang Y, Biswas TK. Effects of Withania somnifera Extract in Chronically Stressed Adults: A Randomized Controlled Trial. Nutrients. 2024;16(9):1293. doi:10.3390/nu16091293
- Akhgarjand C, Asoudeh F, Bagheri A, et al. Does Ashwagandha supplementation have a beneficial effect on the management of anxiety and stress? A systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2022;36(11):4115-4124. doi:10.1002/ptr.7598
- Tóth-Mészáros A, Garmaa G, Hegyi P, et al. The effect of adaptogenic plants on stress: A systematic review and meta-analysis. J Funct Foods. 2023;108:105695. doi:10.1016/j.jff.2023.105695
- Pandit S, Srivastav AK, Sur TK, Chaudhuri S, Wang Y, Biswas TK. Effects of Withania somnifera Extract in Chronically Stressed Adults: A Randomized Controlled Trial. Nutrients. 2024;16(9):1293. doi:10.3390/nu16091293
Ashwagandha in Improving Mental Wellness: A Meta-Analysis of Clinical Trials © 2024 by Sarah Adkins, Mohammad Faisal Hossain is licensed under Attribution 4.0 International
Note
Place of Publication: PSciP Publishing LLC, Oakwood, VA, USA.