Abstract
The prevalence of misbranded and adulterated products in the US dietary supplements (DS) market poses significant health risks to consumers. This review article aims to assess the severity of this issue by analyzing recent data, highlighting the need for enhanced regulations to ensure patient safety. Studies (N=174, Mostly Purchased ONLINE) have shown that approximately 36.6% ± 26.1 (%Mean ± SD) of dietary supplements are adulterated, and 57.5% ± 21.4 (%Mean ± SD) are misbranded. Unwanted ingredients, such as active substances like sildenafil and steroids, have been identified in supplements despite existing monitoring efforts. These ingredients, often found in energy-boosting supplements, contribute to significant health risks. However, there have been positive developments in regulatory oversight by the US FDA, including actively monitoring and analyzing supplements in the market and issuing warning letters to manufacturers based on their findings. These actions reflect a proactive approach to addressing the issue, enhancing consumer safety and confidence in the supplement industry. The findings emphasize the importance of improving surveillance and enforcement measures to protect public health better and ensure only safe products reach the market. The review of misbranded and adulterated US data supplements highlights a significant gap in existing research, emphasizing the need for further large-scale investigation. Comprehensive studies are essential to understand the scope of the issue, enhance consumer safety, and ensure the integrity of data supplement products in the market. Customers of DS can also play a significant role in stopping the promotion of misbranded products by checking information and buying products manufactured in Current Good Manufacturing Practice (cGMP) facilities.
Keywords: Dietary supplements, Quality Assurance, Misbranding, Adulteration, Dietary Supplement Health and Education Act (DSHEA).
Introduction
The DS market in the United States is extensive, with Americans investing billions annually to enhance their daily nutrition through essential nutrients. This market comprises a diverse array of products, including vitamins, minerals, proteins, amino acids, and botanicals [1]. By 2024, the U.S. market is projected to reach $56 billion, with vitamin sales alone expected to amount to $22.8 billion [2]. The rapid growth of e-commerce, along with the increased sales and production of DS, presents opportunities to enhance industry regulation by allocating additional resources [3]. Reports of adulteration, misbranding, and misleading claims are prevalent, threatening consumer health and safety [4],[5],[6].
Over the last five years, the US FDA has addressed numerous concerning incidents involving adulterated or misbranded DS. In recent actions in 2024, the US FDA identified that certain products labeled as tejocote root or Brazil seed contain toxic yellow oleander instead. This poses potentially fatal and/or severe health risks, including neurological, gastrointestinal, and cardiovascular effects. The US FDA has conducted tests on 18 samples and is actively removing these dangerous supplements from the market and advising consumers to avoid them [7]. Additionally, on November 17, 2022, the US FDA issued warnings to seven companies for unlawfully selling DS claiming to treat cardiovascular disease. These unapproved products have not undergone safety or efficacy evaluations, prompting the US FDA to give the companies a 15-day deadline to respond or face legal repercussions [8]. Earlier, on May 9, 2022, the US FDA issued warning letters to companies selling adulterated DS containing unapproved ingredients like 5-alpha-hydroxy-laxogenin, higenamine, hordenine, and octopamine, which are marketed as drugs without US FDA approval, risking harm to consumers [9]. These actions underscore the US FDA’s ongoing efforts to protect public health and regulate the supplement industry effectively.
The efficacy and safety of many of these supplements remain debatable, with instances of toxic components being detected, whether intentionally added or not. In response to these challenges, the Dietary Supplement Health and Education Act (DSHEA) of 1994 was established to define dietary supplements and regulate their production and marketing [10]. Unlike drugs, dietary supplements do not require pre-approval by the US government for safety or efficacy, and the labeling is not reviewed by the US Food and Drug Administration (FDA) before distribution. Under the DSHEA, manufacturers, and distributors are prohibited from marketing adulterated or misbranded products, and they are responsible for ensuring product safety and compliance with federal regulations. The US FDA oversees the regulation of finished DS products and dietary ingredients, taking action against any adulterated or misbranded products after they enter the market [11]. This article aims to highlight the widespread issue of adulteration and misbranding in U.S. dietary supplements and natural products, emphasizing the associated health risks. By examining the factors contributing to these issues, such as regulatory gaps and enforcement challenges, the article seeks to empower consumers to recognize and avoid adulterated and misbranded products. Additionally, it advocates for stricter regulations and improved enforcement mechanisms to ensure the safety and authenticity of DS in the market.
Method
This review article utilized PubMed and Google Scholar to gather relevant literature on misbranded and adulterated products in the DS market. The search terms included “misbranded adulterated dietary supplements,” “US FDA regulations,” and related keywords. The initial screening involved reviewing titles and abstracts to identify potentially suitable publications. Selected articles were then thoroughly reviewed to extract relevant information for this comprehensive analysis. To ensure a thorough review, both full-text articles and abstracts were considered.
Results and Discussion
In this study, we conducted a detailed analysis of several studies published on misbranded and adulterated products, as well as research on the identification of toxic and unwanted ingredients in DS. Additionally, we reviewed warning letters issued over the past five years to gain insight into the regulatory landscape of the industry. Table 1 summarizes seven studies conducted between 2016 and 2024, focusing on various types of supplements such as weight loss, sports performance, brain health, and general health products. These studies revealed a concerning trend (Fig 1).
Table 1: Summary of Literature on Misbranded and Adulterated Products in the DS Market [4],[5],[12],[13],[14],[15],[16].
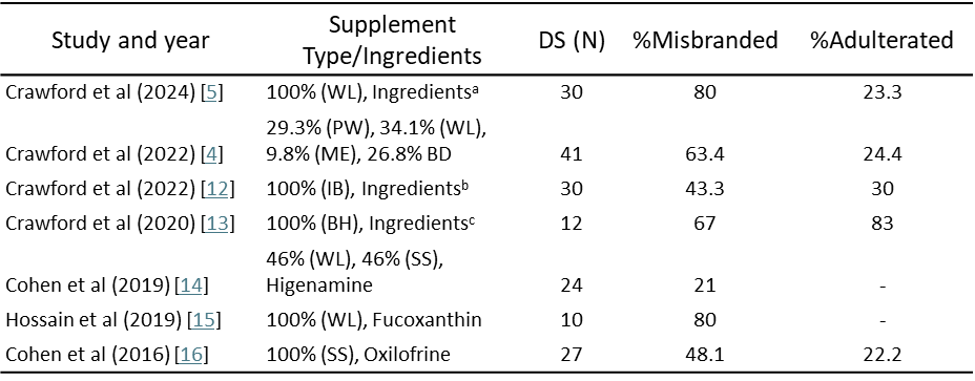
N: the total number of product analyses; DS: Dietary Supplement; WL: Weight Loss; PW: Pre-Workout products; ME: Male Enhancement/testosterone boosters; BD: Body Building; SS: Sports Supplement; MB: Memory Boosting; AS: Androgenic Steroids; BH: Brain Health; IB: Immune Boosting
aBesides caffeine, other common ingredients listed on labels included green tea extract (17 products), yohimbe/yohimbine (15 products), black pepper (13 products), L-tyrosine (12 products), grains of paradise (6-paradol) (12 products), acetyl-L-carnitine (10 products), huperzine A (10 products), Citrus aurantium [standardized for p-synephrine] or synephrine (10 products), Rauwolfia/rauwolscine (8 products), and raspberry ketones (7 products).
bThe most frequently used products were echinacea (14 products), elderberry (18 products), vitamin C (24 products), vitamin D (15 products), and zinc (25 products)
cCommonly reported herbal ingredients were Bacopa leaf (9/12) and Huperzine A (10/12) extracts. Other frequently mentioned ingredients were phosphatidylserine (8/12), vinpocetine (5/12), and coffee extracts (5/12).
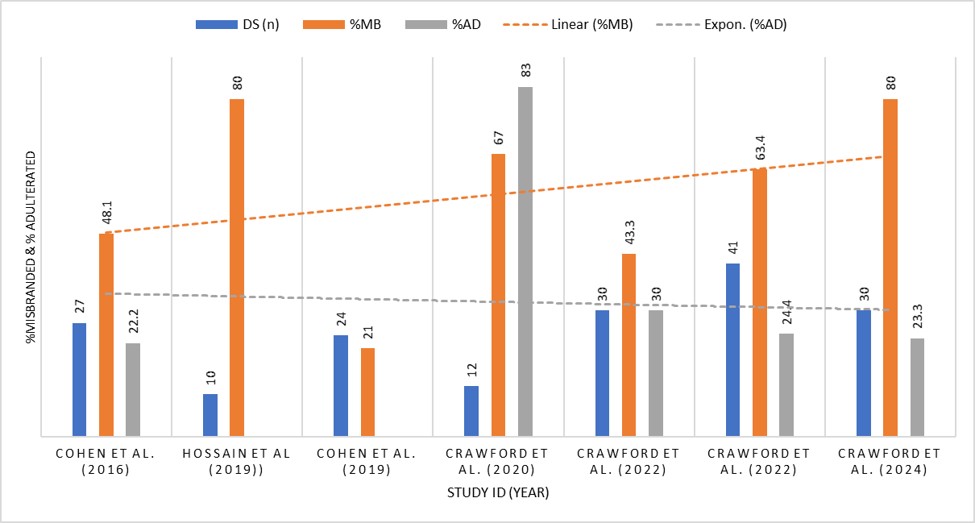
Figure 1: Bar Diagram and Trends Over Time (%Misbranded and %Adulterated DS in the USA)
In Figure 1, The bar or trend diagram, covering 7 studies, focused on the percentage of misbranded and adulterated DS products in the United States from 2016 to 2024. The analysis revealed a concerning increase in misbranded DS over the years. The regression analysis showed a clear upward trend in misbranded products, emphasizing the urgent need for stronger regulatory measures in the expanding supplement market. Despite this, a slight downward trend in adulterated product consumption was noted, which can be seen as a positive sign. Nevertheless, further research is essential, as only a few studies have thoroughly examined this critical area.
Various studies have highlighted significant inconsistencies and potential risks associated with DS. Analysis using UPLC-MS/MS revealed that the sympathomimetic compound 1,3-Dimethylamylamine (DMAA) in supplements is synthetic and not derived from Geranium or Pelargonium plants [17]. One Research indicates that over nine months, popular sports supplements displayed considerable variations in their caffeine and other stimulant content [18]. In another study, Piracetam, which is not US FDA-approved, was found in significant amounts in multiple brands. The adverse effects of Piracetam are anxiety, insomnia, agitation, depression, drowsiness, and weight gain [19]. Also, weight loss and sports supplements labeled with deterenol are associated with cardiac arrest [20]. An investigation in New York found that residents experiencing vague and nonspecific health issues, such as fatigue, hair loss, and muscle aches, were linked to B-50 vitamin and multi-mineral supplements containing androgenic steroids. This highlights the necessity for stricter regulatory measures [21]. Finally, a study of adverse events reported to major U.S. marketers over 2.5 years found 203 serious adverse events, primarily related to weight loss and glycemic control supplements, highlighting the necessity for further research into the safety of DS [22]. While some articles mentioned the presence of unwanted ingredients, many products were found to contain toxic substances not listed on their labels. A quality improvement study analyzing US FDA warnings from 2007 to 2016 found that 776 DS contained unapproved pharmaceutical ingredients. These products, often marketed for sexual enhancement, weight loss, or muscle building, commonly included sildenafil, sibutramine, and synthetic steroids. Notably, 20.2% of these products had more than one unapproved ingredient [23]. This discrepancy raises serious concerns about consumer safety and the need for stricter regulations to ensure product quality and transparency. The analysis in this article further highlighted the need for proper oversight and monitoring in the dietary supplement market.
Conclusion
The widespread issues of misbranding and adulteration in DS in the US market highlight the urgent need for more rigorous regulations. Recent cases involving toxic ingredients and unapproved products underscore the necessity for improvement. Strengthening the Dietary Supplement Health and Education Act (DSHEA) by implementing measures such as mandatory product registration, enhanced enforcement, improved adverse event reporting, and quality certification programs can greatly enhance the safety and integrity of DS. Adopting stronger regulations will help combat the spread of harmful products and protect consumers from potential health risks associated with misbranded and adulterated supplements. Additionally, consumers can contribute significantly to stopping the promotion of misbranded products by verifying details like manufacturer information and supplement facts and by purchasing products made in cGMP facilities from reliable stores. Consumers should be aware that dietary supplements are not drugs and should not be promoted as cures for diseases. Misleading information from manufacturers claiming their supplements can cure or treat illnesses can be fraudulent. For consumers seeking to make informed decisions when purchasing dietary supplements (Mainly from Online Stores), it is crucial to verify the information provided by the manufacturer.
Study Limitations
The review article on misbranded and adulterated dietary supplements is limited by the use of only 7 articles from 2016 to 2024 for trend analysis, and the majority of the supplements included in the studies were purchased online. To ensure a more accurate assessment of trends, further extensive research is necessary.
Authors’ Contributions
Each author actively engaged in analyzing and discussing the results, thereby contributing to the finalization of the manuscript. SA and MB conducted an in-depth literature review, of equal contributions. MFH provided supervision throughout the article, ensuring a comprehensive interpretation of the findings.
Conflict of interest
The authors declare no conflict of interest.
References
- Djaoudene O, Romano A, Bradai YD, et al. A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects. Nutrients. 2023;15(15):3320. doi:10.3390/nu15153320
- Jiao L, Chan P. IS IT TIME TO REGULATE THE DIETARY SUPPLEMENT INDUSTRY? J Acad Bus Econ. 2020;20(2):61-74.
- Starr RR. Too Little, Too Late: Ineffective Regulation of Dietary Supplements in the United States. Am J Public Health. 2015;105(3):478-485. doi:10.2105/AJPH.2014.302348
- Crawford C, Walter AR, Avula B, et al. Relative safety and quality of various dietary supplement products U.S. Service Members ask about. Clin Toxicol Phila Pa. 2022;60(6):737-744. doi:10.1080/15563650.2022.2036751
- Crawford C, Avula B, Lindsey AT, Katragunta K, Khan IA, Deuster PA. Label Accuracy of Weight Loss Dietary Supplements Marketed Online With Military Discounts. JAMA Netw Open. 2024;7(5):e249131. doi:10.1001/jamanetworkopen.2024.9131
- Cohen PA, Avula B, Khan I. The unapproved drug centrophenoxine (meclofenoxate) in cognitive enhancement dietary supplements. Clin Toxicol. 2022;60(10):1156-1158. doi:10.1080/15563650.2022.2109485
- Nutrition C for FS and A. FDA Issues Warning About Certain Supplements Substituted with Toxic Yellow Oleander (January 2024). FDA. Published online June 5, 2024. Accessed July 17, 2024. https://www.fda.gov/food/alerts-advisories-safety-information/fda-issues-warning-about-certain-supplements-substituted-toxic-yellow-oleander-january-2024
- Nutrition C for FS and A. FDA Issues Warning Letters to Companies Selling Dietary Supplements that Claim to Treat Cardiovascular Disease. FDA. Published online August 15, 2023. Accessed July 17, 2024. https://www.fda.gov/food/cfsan-constituent-updates/fda-issues-warning-letters-companies-selling-dietary-supplements-claim-treat-cardiovascular-disease
- Nutrition C for FS and A. FDA Sends Warning Letters to Multiple Companies for Illegally Selling Adulterated Dietary Supplements. FDA. Published online January 19, 2024. Accessed July 17, 2024. https://www.fda.gov/food/cfsan-constituent-updates/fda-sends-warning-letters-multiple-companies-illegally-selling-adulterated-dietary-supplements
- Office of Dietary Supplements – Dietary Supplement Health and Education Act of 1994. Accessed July 21, 2024. https://ods.od.nih.gov/About/DSHEA_Wording.aspx
- Nutrition C for FS and A. Dietary Supplements. FDA. Published February 16, 2024. Accessed July 21, 2024. https://www.fda.gov/food/dietary-supplements
- Crawford C, Avula B, Lindsey AT, et al. Analysis of Select Dietary Supplement Products Marketed to Support or Boost the Immune System. JAMA Netw Open. 2022;5(8):e2226040. doi:10.1001/jamanetworkopen.2022.26040
- A Public Health Issue: Dietary Supplements Promoted for Brain Health and Cognitive Performance | The Journal of Alternative and Complementary Medicine. Accessed July 21, 2024. https://www.liebertpub.com/doi/full/10.1089/acm.2019.0447
- Cohen PA, Travis JC, Keizers PHJ, Boyer FE, Venhuis BJ. The stimulant higenamine in weight loss and sports supplements. Clin Toxicol Phila Pa. 2019;57(2):125-130. doi:10.1080/15563650.2018.1497171
- Ek T. Evaluation of Fucoxanthin Content in Popular Weight Loss Supplements: The Case for Stricter Regulation of Dietary Supplements. doi:10.23937/2572-4010.1510031
- Pharmaceutical doses of the banned stimulant oxilofrine found in dietary supplements sold in the USA – Cohen – 2017 – Drug Testing and Analysis – Wiley Online Library. Accessed July 21, 2024. https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/full/10.1002/dta.1976
- Austin KG, Travis J, Pace G, Lieberman HR. Analysis of 1,3 dimethylamylamine concentrations in Geraniaceae, geranium oil and dietary supplements. Drug Test Anal. 2014;6(7-8):797-804. doi:10.1002/dta.1491
- Attipoe S, Cohen PA, Eichner A, Deuster PA. Variability of Stimulant Levels in Nine Sports Supplements Over a 9-Month Period. Published online October 1, 2016. doi:10.1123/ijsnem.2015-0177
- 19. Cohen PA, Zakharevich I, Gerona R. Presence of Piracetam in Cognitive Enhancement Dietary Supplements. JAMA Intern Med. 2020;180(3):458-459. doi:10.1001/jamainternmed.2019.5507
- Cohen PA, Travis JC, Vanhee C, Ohana D, Venhuis BJ. Nine prohibited stimulants found in sports and weight loss supplements: deterenol, phenpromethamine (Vonedrine), oxilofrine, octodrine, beta-methylphenylethylamine (BMPEA), 1,3-dimethylamylamine (1,3-DMAA), 1,4-dimethylamylamine (1,4-DMAA), 1,3-dimethylbutylamine (1,3-DMBA) and higenamine. Clin Toxicol. 2021;59(11):975-981. doi:10.1080/15563650.2021.1894333
- Tran BN, Okoniewski R, Spink BC, LeMaster DM, Aldous KM, Spink DC. Androgenic steroids in Over-the-Counter dietary Supplements: Analysis for association with adverse health effects. Steroids. 2023;193:109199. doi:10.1016/j.steroids.2023.109199
- Schmitz MD M Stephen M, Lopez MD M Hector L, Mackay ND D, Nguyen BS H, Miller BS PE. Serious Adverse Events Reported with Dietary Supplement Use in the United States: A 2.5 Year Experience. J Diet Suppl. 2020;17(2):227-248. doi:10.1080/19390211.2018.1513109
- 23. Tucker J, Fischer T, Upjohn L, Mazzera D, Kumar M. Unapproved Pharmaceutical Ingredients Included in Dietary Supplements Associated With US Food and Drug Administration Warnings. JAMA Netw Open. 2018;1(6):e183337. doi:10.1001/jamanetworkopen.2018.3337
Compromising Health: Review of Misbranded and Adulterated US Dietary Supplements © 2024 by Sartaz Araf, Moin Bhuiyan, Mohammad Faisal Hossain is licensed under Attribution 4.0 International
Note
Place of Publication: PSciP Publishing LLC, Oakwood, VA, USA.