Abstract
Osteoarthritis (OA) is an extremely prevalent degenerative disease affecting 32.5 million people in the United States and 595 million people worldwide. Adults aged 40 and older are primarily affected by OA, with it being ranked seventh among causes of years lived with disability (YLDs). OA is commonly treated with over-the-counter pain medications, but these are not always completely effective. Because of the high prevalence of OA and the lack of satisfaction with the available treatment, a lot of individuals feel the need to seek out dietary supplements as a subsidiary treatment. Dietary supplements are commonly used, with 57.6% of adults aged 20 and over using at least one. This Systematic Review (SR) and Meta-Analysis aims to examine the literature available on glucosamine (GA) and chondroitin (CD) to provide an unbiased overview of the efficacy and safety of these supplements. Ten randomized controlled trials were reviewed for the systematic review. Three of those articles were then statistically analyzed for pain and function endpoints in the meta-analysis. While the results were not statistically significant, a slight positive trend was observed. (GA+CD) were found to have minimal adverse effects. (GA+CD) may help with osteoarthritis pain and function, but it is advisable to consult with a healthcare provider before initiating supplementation.
Keywords: Glucosamine, chondroitin, osteoarthritis, dietary supplement, A Systematic Review and Meta-Analysis.
Introduction
Osteoarthritis (OA) is a degenerative disease that causes the breakdown of cartilage in the joint space, leading to pain, stiffness, tenderness, and loss of function [1]. It is an extremely common disease, affecting approximately 32.5 million people in the United States [2], and 595 million people worldwide [3]. The prevalence of OA increases with age, ranking seventh among reasons people aged 70+ years lived with disability [3]. Due to the prevalence of the disease, people resort to looking for solutions other than prescribed medication. This leads individuals to seek out dietary supplements. Per a study done via the CDC in 2021 [4], 57.6% of adults aged 20 and over used at least 1 dietary supplement in the last 30 days.
Glucosamine is a molecule naturally found in your body and is theorized to play a role in slowing cartilage breakdown via maintaining elasticity, strength, and resilience of cartilage, as well as inhibiting proteolytic enzymes and promoting glycosaminoglycan and proteoglycan synthesis. It also has anti-inflammatory, immunosuppressive, and anti-catabolic properties [5]. GA can be sourced naturally via extraction from shells or shellfish or made in a lab [6]. There are three commercially available forms of GA: GA HCl, G sulfate, and N-acetyl GA. (GA) has a pKa of 7.58, a logP of -2.8, MW of 179.17 g/mol, and oral bioavailability of 44% [7]. (CD) is another molecule that is naturally found in your body. It is theorized to slow the progression and modify the course of OA [8]. (C) can be naturally sourced via extraction from shark and bovine cartilage or made in a lab [9]. (CD) has a pKa of 1.5-2, a logP of -4.8, MW of 463.37 g/mol, and oral bioavailability of 10-20% [10].
According to the FDA, “Dietary supplements are intended to add to or supplement the diet and are different from conventional food” [11]. However, dietary supplements are not regulated in the same way as drug products. They are regulated by a branch of the US FDA called the Center for Food Safety and Applied Nutrition [12]. Dietary supplements are monitored for misbranding and adulteration, not efficacy [13]. In other words, they are monitored for inaccurate levels and claims, not for if they work. It is the responsibility of the manufacturers and distributors to analyze their products for safety and efficacy [12]. The goal of this SR & MA is to evaluate the available data on dietary supplements (GA + CD) for efficacy and safety and provide information so that the readers can make informed decisions on whether or not to recommend these supplements.
Method
A SR & MA was performed using the electronic database PubMed. The keywords (glucosamine, dietary supplement, osteoarthritis) were used and the results were then evaluated for appropriateness as well as the availability of full text. The search included articles published between 2000 and 2024. Specifications were made only to review randomized controlled trials. Ten articles were evaluated for the systematic review, and only 3 were analyzed in the meta-analysis. This was due to a lack of consistency between the articles and their reported data. An effort was made only to include articles with the same dose of (GA + CD) (1500 mg GA and 1200 mg CD). However, there were two articles in which the doses were different.
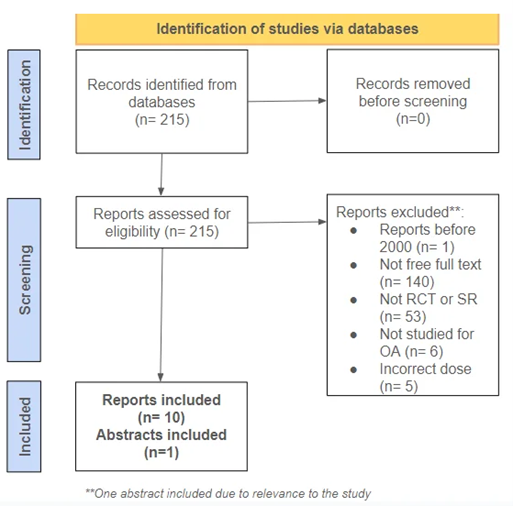
Figure 1: Flow Chart of Study Selection
Results and Discussion
Efficacy/Effectiveness:
SRs are when researchers evaluate all relevant studies on a particular topic and form their conclusions and summarization of the data [14]. MAs are more specific in the topic of discussion [15]. MAs also differ from SRs in that researchers do their statistical analysis of the data, typically involving heterogeneity, effect size, forest plots, and funnel plots. The purpose of heterogeneity is to determine how consistent the study is. This is evaluated with I2 – if the I2 value is >50%, the study might be inconsistent for reasons other than chance and needs to be evaluated [16]. On the contrary, an I2 value <50% means the study is homogenous and the studies had consistent results [17]. “Cohens d is a standardized effect size for measuring the difference between two group means” [18]. Cohen’s d is evaluated based on three categories: small (d = 0.2), medium (d = 0.5), and large (d = 0.8). These three categories refer to the effect of the treatment compared to the placebo. In the instance of this study, cohen’s d was used to evaluate the difference between the treatment group and the control group. A forest plot is used in meta-analyses as a way to summarize the conclusions of relevant studies on a particular data point [16].
Table 1: Summary of the included studies [25, 26, 27, 28, 29, 30, 31, 32, 33, 34].
| Author, Year | Dose | Duration | Participants | % Weight | Outcomes |
| Osama et al, 2022 | G 500 mg TID + C 400 mg TID | 4 weeks | 24 | 0.93 | ⛔ |
| Kılıç, 2021 | 750 mg G + 600 mg C + 350 mg MSM at 2×1 doses | 3 months | 26 | 1.01 | ⛔ |
| Wang et al, 2021* | 750 mg G + 250 mg C + 50 mg HA | 8 weeks | 80 | 3.11 | ⛔ |
| Lubis et al, 2017 | 1500 mg G + 1200 mg C vs 1500 mg G + 1200 mg C + 500 mg MSM vs. placebo | 3 months | 147 | 5.72 | ✅ |
| Sterzi et al, 2016 | 500 mg G + 400 mg C + 200 mg MSM at 2×1 doses | 8 weeks | 53 | 2.06 | ✅ |
| Lugo et al, 2016* | 1500 mg G + 1200 mg C vs. 40 mg UC-II vs. placebo | 180 days | 191 | 7.43 | ✅ |
| Navarro et al., 2015 | 1500 mg G + 1200 mg C | 28 days | 20 | 0.78 | ✅ |
| Hochberg et al., 2008 | 1500 mg G vs. 1200 C vs. G+C vs. 200 mg celecoxib | 24 weeks | 1583 | 61.60 | ✅ |
| Sawitzke et al., 2008 | 1500 mg G vs. 1200 C vs. G+C vs. 200 mg celecoxib | 24 months | 357 | 13.89 | ⛔ |
| Messier et al, 2007* | 1500 mg G + 1200 mg Chondroitin | 12 months | 89 | 3.46 | ⛔ |
*Articles are ones reviewed in MA
The Western Ontario and McMaster Universities Arthritis Index (WOMAC) score is commonly used for the evaluation of OA. The test has three different categories: pain, function, and stiffness. For the meta-analyses of (GA + CD), pain and function were evaluated. There was a lack of agreement between the various studies. Upon first evaluation, there are more negative studies than positive ones. However, the cumulative percent weight of the positive studies is 77.59%.
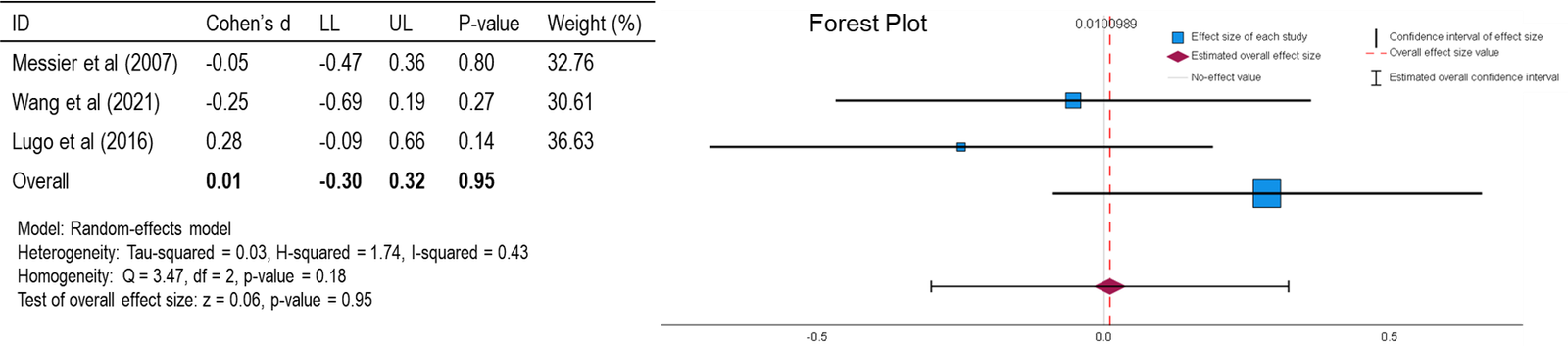
Figure 2: Forest Plot of Pain (WOMAC) [27, 30, 34].
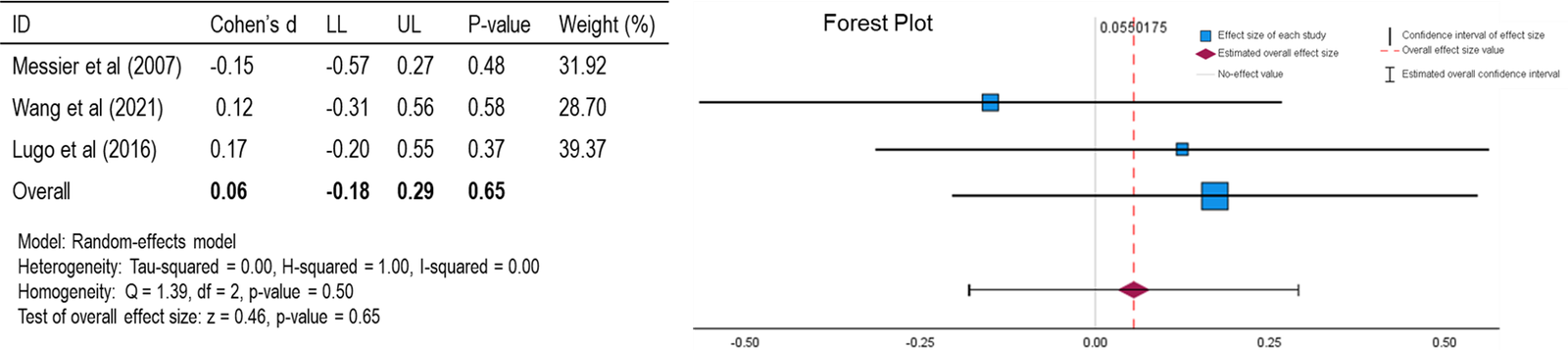
Figure 3: Forest Plot of Function (WOMAC) [27, 30, 34].
For both forest plots (Figures 2 & 3), the mean is close to the null effect with a slight positive correlation. This suggests that, on average, there is not a strong effect of (GA + CD) on pain or function, but there might be a minimal positive effect observed. An I2 value of 0.43 and 0.00 for pain and function (P + F) respectively, suggests moderate heterogeneity in the study results, meaning that there is some variability in the effect sizes across the included studies, but not enough to be concerning. With a p-value of 0.95 and 0.65 for (P + F), respectively, the findings are not statistically significant. This means the observed results are likely due to random chance rather than a true effect of (GA + CD) on (P + F). Cohen’s d is a measure of effect size that indicates the standardized difference between two means. In this case, Cohen’s d of 0.01 and 0.06 for (P + F), respectively, is very small, suggesting that the effect of (GA + CD) on (P + F) is minimal. In summary, the forest plot shows that the overall effect of (GA + CD) on (P + F) is not statistically significant, with a small positive correlation and a very small effect size according to Cohen’s d. The homogeneity within the analysis indicates that the results are reasonably consistent across the included studies.
The articles did not all have the same results. However, after careful review and evaluation, there is enough evidence to say that (GA + CD) supplementation may provide benefits. These supplements seem to have more of an effect on slowing the progression of diminishing joint space rather than controlling pain. For pain and function evaluations, (GA + CD) only worked marginally better than placebo. For this article, (GA + CD) was explored for the treatment of osteoarthritis. Osteoarthritis becomes more common with age, so these supplements are recommended for older adults (40+).
Drug Interactions
It is widely believed by the general public that dietary supplements are safe and, therefore, have no drug interactions because they are sold over the counter. This, however, is not factual and can be harmful to individuals who believe this. Dietary supplements can cause harm in several ways, including interacting with prescription medication, interfering with lab work, or having adverse effects during surgery [11]. (GA) or the combination of (GA + CD) should be avoided in patients who are on warfarin. (GA) or the combination of (GA + CD) can cause increased INR [19]. Additionally, (GA) is theorized to reduce the efficacy of acetaminophen [20]. However, a CYP inhibition assay was performed and (GA + CD) was not found to have inhibitory effects on CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2C19, CYP2D6, and CYP3A4 [21]. (CD) itself does not appear to have any individual drug interactions other than the synergistic effect it may have on (GA)’s interaction with warfarin.
Adverse Effects and Risk
Overall, (GA + CD) does not have an abundance of adverse effects. They both can cause mild side effects such as bloating, nausea, diarrhea, and constipation [22,23]. Due to the sourcing process of both (GA + CD), these supplements should not be used in individuals with a shellfish allergy. However, some available forms do not contain shellfish; patients should consult their doctor or pharmacist to determine which form is right for them. Also, caution should be used if using the HCl form of (GA). The HCl form of (GA) contains salt which may interfere with blood pressure and renal function in patients” [24].
Conclusion
(GA + CD) shows potential to supplement the treatment of osteoarthritis. After reviewing the relevant and available literature, we determined that while the data for these supplements is not statistically significant, the positive trend suggests that supplementation may slow disease progression in individuals who suffer from osteoarthritis. Exclusively analyzing randomized controlled trials enhanced the quality and reliability of this SR/MA. However, these supplements should not replace prescribed medications. These supplements are not approved by the FDA, so individuals seeking to take these supplements should consult with their healthcare provider. When reviewing the available articles for this analysis, there was an apparent lack of research on (GA + CD) when used alone. There were a couple of studies available, but they used small sample sizes. There needs to be further research on both (GA + CD) alone to examine how the effects differ from when they are used in combination. This could be managed by analyzing (GA) or (CD) as additional treatment in individuals already using medication therapy to treat their OA.
Conflict of interest
The authors declare no conflict of interest.
References
- Osteoarthritis-Osteoarthritis – Symptoms & causes. Mayo Clinic. Accessed February 23, 2024.
- Osteoarthritis | CDC. Published June 13, 2023. Accessed February 23, 2024. [
- The Lancet: New study reveals the most common form of arthritis, osteoarthritis, affects 15% of the global population over the age of 30 | Institute for Health Metrics and Evaluation. Accessed February 23, 2024.
- Products – Data Briefs – Number 399 – February 2021. doi:10.15620/cdc:101131
- Glucosamine Sulfate – an overview | ScienceDirect Topics. Accessed February 23, 2024.
- Mayo Clinic. Accessed February 23, 2024.
- D-Glucosamine. Accessed February 23, 2024. [
- Jerosch J. Effects of Glucosamine and Chondroitin Sulfate on Cartilage Metabolism in OA: Outlook on Other Nutrient Partners Especially Omega-3 Fatty Acids. Int J Rheumatol. 2011;2011:969012. doi:10.1155/2011/969012
- Chondroitin Information | Mount Sinai – New York. Accessed February 23, 2024.
- Chondroitin 4-sulfate. Accessed February 23, 2024.
- Commissioner O of the. FDA 101: Dietary Supplements. FDA. Published online June 9, 2022. Accessed February 23, 2024.
- Nutrition C for FS and A. Questions and Answers on Dietary Supplements. FDA. Published online February 21, 2024. Accessed February 27, 2024.
- Nutrition C for FS and A. Dietary Supplements. FDA. Published February 16, 2024. Accessed February 23, 2024.
- Uman LS. Systematic Reviews and Meta-Analyses. J Can Acad Child Adolesc Psychiatry. 2011;20(1):57-59.
- IBM Documentation. Published August 4, 2023. Accessed February 23, 2024.
- Cantley N. Tutorial: How to read a forest plot. Students 4 Best Evidence. Published July 11, 2016. Accessed February 23, 2024.
- Forest plot at a glance | Cochrane UK. Accessed February 23, 2024.
- Cohens D: Definition, Using & Examples – Statistics By Jim. Accessed February 23, 2024.
- Knudsen JF, Sokol GH. Potential glucosamine-warfarin interaction resulting in increased international normalized ratio: case report and review of the literature and MedWatch database. Pharmacotherapy. 2008;28(4):540-548. doi:10.1592/phco.28.4.540
- Bush TM, Rayburn KS, Holloway SW, et al. Adverse interactions between herbal and dietary substances and prescription medications: a clinical survey. Altern Ther Health Med. 2007;13(2):30-35.
- Kim SM, Jo SY, Park HY, Lee YR, Yu JS, Yoo HH. Investigation of Drug-Interaction Potential for Arthritis Dietary Supplements: Chondroitin Sulfate, Glucosamine, and Methylsulfonylmethane. Molecules. 2023;28(24):8068. doi:10.3390/molecules28248068
- GLUCOSAMINE: Overview, Uses, Side Effects, Precautions, Interactions, Dosing and Reviews. Accessed February 23, 2024.
- CHONDROITIN SULFATE: Overview, Uses, Side Effects, Precautions, Interactions, Dosing and Reviews. Accessed February 23, 2024.
- Henrotin Y, Mobasheri A, Marty M. Is there any scientific evidence for the use of glucosamine in the management of human osteoarthritis? Arthritis Res Ther. 2012;14(1):201. doi:10.1186/ar3657
- Muhammad Osama, Babur MN, Siddiqi FA, Tassadaq N, Tareen MAA. Effects of glucosamine and chondroitin sulfate supplementation in addition to resistance exercise training and manual therapy in patients with knee osteoarthritis: a randomized controlled trial. J Pak Med Assoc. Published online January 29, 2022. doi:10.47391/JPMA.2444
- Cömert Kılıç S. Does glucosamine, chondroitin sulfate, and methylsulfonylmethane supplementation improve the outcome of temporomandibular joint osteoarthritis management with arthrocentesis plus intraarticular hyaluronic acid injection. A randomized clinical trial. J Cranio-Maxillofac Surg. 2021;49(8):711-718. doi:10.1016/j.jcms.2021.02.012
- Wang SJ, Wang YH, Huang LC. Liquid combination of hyaluronan, glucosamine, and chondroitin as a dietary supplement for knee osteoarthritis patients with moderate knee pain. Medicine (Baltimore). 2021;100(40):e27405. doi:10.1097/MD.0000000000027405
- Lubis AMT, Siagian C, Wonggokusuma E, Marsetyo AF, Setyohadi B. Comparison of Glucosamine-Chondroitin Sulfate with and without Methylsulfonylmethane in Grade I-II Knee Osteoarthritis: A Double Blind Randomized Controlled Trial. Acta Medica Indones. 2017;49(2):105.
- The efficacy and safety of a combination of glucosamine hydrochloride, chondroitin sulfate and bio-curcumin with exercise in the treatment of knee osteoarthritis: a randomized, double-blind, placebo-controlled study – European Journal of Physical and Rehabilitation Medicine 2016 June;52(3):321-30. Accessed February 26, 2024.
- Lugo JP, Saiyed ZM, Lane NE. Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: a multicenter randomized, double-blind, placebo-controlled study. Nutr J. 2016;15:14. doi:10.1186/s12937-016-0130-8
- Navarro SL, White E, Kantor ED, et al. Randomized trial of glucosamine and chondroitin supplementation on inflammation and oxidative stress biomarkers and plasma proteomics profiles in healthy humans. PloS One. 2015;10(2):e0117534. doi:10.1371/journal.pone.0117534
- Hochberg MC, Clegg DO. POTENTIAL EFFECTS OF CHONDROITIN SULFATE AND JOINT SWELLING: A GAIT REPORT. Osteoarthr Cartil OARS Osteoarthr Res Soc. 2008;16(0 3):S22-S24. doi:10.1016/j.joca.2008.06.024
- Sawitzke AD, Shi H, Finco M, et al. The Effect of Glucosamine and/or Chondroitin Sulfate on the Progression of Knee Osteoarthritis: A GAIT Report. Arthritis Rheum. 2008;58(10):3183-3191. doi:10.1002/art.23973
- Messier SP, Mihalko S, Loeser RF, et al. Glucosamine/chondroitin combined with exercise for the treatment of knee osteoarthritis: a preliminary study. Osteoarthritis Cartilage. 2007;15(11):1256-1266. doi:10.1016/j.joca.2007.04.016
Evaluating the Efficacy of Glucosamine and Chondroitin for the Supplemental Treatment of Osteoarthritis: A Systematic Review and Meta-Analysis © 2024 by Janessa Harris, McKinley Webb, Mohammad Faisal Hossain is licensed under CC BY 4.0
Note
Place of Publication: PSciP Publishing LLC, Oakwood, VA, USA.