Abstract
Migraine, a neurological condition, affects approximately 1.1 billion people worldwide, making it the third leading cause of disability. It significantly impacts quality of life and incurs an annual economic burden exceeding $78 billion in the United States. This systematic review and meta-analysis aims to evaluate the efficacy of Feverfew Dietary Supplement in migraine prophylaxis, with migraine frequency considered as the primary outcome. Three trials (n=237) were included in the meta-analysis (MA) of migraine frequency, and an additional three trials were synthesized narratively in the systematic review. In the MA, feverfew showed a non-significant reduction in migraine frequency compared to placebo (Overall effect size, Cohen's d: -0.19; 95% CI: -0.52 to 0.14; I² = 34%, p = 0.26). These findings suggest that Feverfew might offer some benefit in individual patients, but given the small, non-significant effect size, it should not be recommended as a primary prophylactic agent over established treatments. Feverfew may not be effective as a standalone prophylactic supplement for migraines; however, its potential in combination therapies seems promising and requires further evaluation. Larger trials with standardized parthenolide content and extended durations are necessary to clarify feverfew's definitive role in migraine prophylaxis.
Keywords: Feverfew, Tanacetum parthenium, Parthenolide, Migraine Prophylaxis, Meta-Analysis.
Introduction
Headache disorders are a significant global health concern, affecting approximately 40% of the population (3.1 billion people in 2021), with a higher prevalence in females and consistent impact across nearly all age groups from childhood to old age, according to the World Health Organization [1]. In the United States, an estimated 15.3% of adults (1 in 6 adults) experience migraines,18% of women (1 in 5 women) compared to 6% of men, potentially due to hormonal effects [2]. The total national cost in the U.S was 78 billion dollars, including direct healthcare expenditures and indirect expenses through reduced productivity due to pain, nausea, and photophobia [3], whereas according to the 2018 study by Bonafede et al., individuals with migraine incurred an additional $8,924 annually compared to non-migraine controls, including approximately $6,575 in direct medical costs and $2,350 in indirect costs [4]. Many herbal products, such as Feverfew, Butterbur, and Ginger, are commonly used as prophylactic agents for migraines [5]. Food can play a significant role in migraine by reducing triggers, and maintaining regular eating patterns can help decrease migraine frequency. Generally, food that contains tyramine as alcohol (especially red wine), aged cheeses, processed meats, and chocolate, can trigger migraine [6]. On the other hand, a diet rich in magnesium (Leafy greens, avocados) and limiting processed foods and saturated fats may decrease the frequency of migraine attacks [7]. Feverfew, a perennial herb in southeastern Europe but now cultivated in North America, has been used for centuries to treat headaches, arthritis, and dermatitis [8]. The primary active substance, parthenolide (a sesquiterpene lactone), has an anti-migraine mechanism of action by inhibiting prostaglandin synthesis, thus reducing vascular smooth muscle spasms, and decreasing platelet granule secretion [9]. Feverfew is available in many forms as capsules, tablets, or liquid extracts. There is a challenge in the instability of parthenolide, as it degrades over time during storage, resulting in commercial products with decreased efficacy [10]. Several clinical trials discussing feverfew’s role in migraine prophylaxis have reported variable findings in migraine attack frequency, providing a potential for further studies [11] [12]. In the U.S, feverfew is classified as a dietary supplement under the Dietary Supplement Health and Education Act of 1994 (DSHEA), which obligates manufacturers to ensure product safety and accurate labeling without requiring pre-market approval from the FDA [13]. The objective of this systematic review and meta-analysis is to evaluate the efficacy of feverfew supplementation in migraine prophylaxis by getting evidence from clinical trials, with a focus on migraine frequency as the primary outcome measure.
Method
This systematic review was performed to evaluate the effectiveness of feverfew supplementation for migraine prophylaxis using PubMed and Google Scholar as the main sources of data. It focused on randomized controlled clinical trials. Initially, 187 records were identified matching the broad scope of the search,112 records were excluded, and 75 reports were identified for full-text eligibility assessment. After a detailed review, 6 reports were matched for inclusion criteria. The duration of the studies ranged from four months to nine months. Three clinical trials were selected for the MA due to their consistent reporting of migraine frequency, while all 6 contributed to the systematic review. A total of 237 participants were included in the selected three trials.
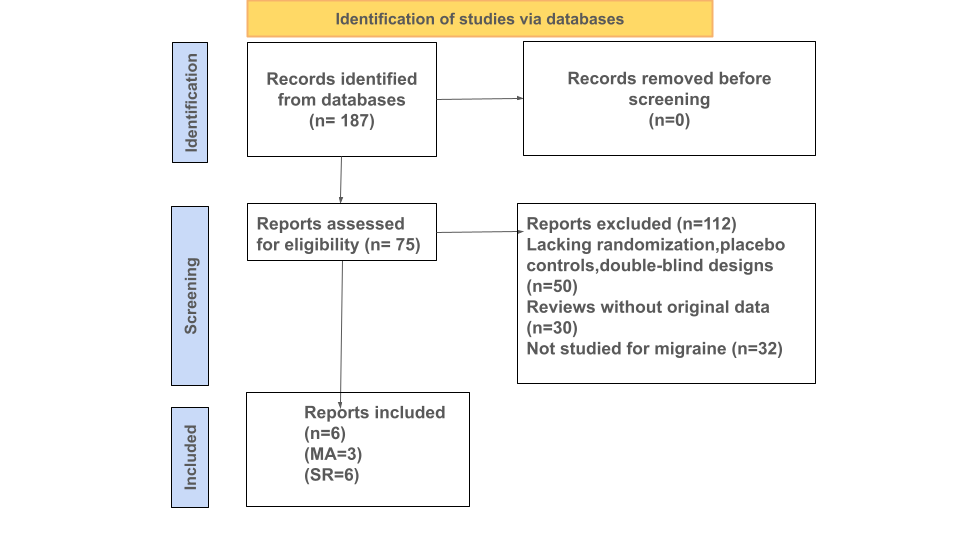
Figure 1: Flow Chart and Study Selection showing the number of articles identified and included in this study.
Results and Discussion
This MA included 3 randomized controlled trials (RCTs) focusing on the frequency of migraine attacks as the primary outcome: Johnson et al. (1985) [14], De Weerdt et al. (1996) [15], and Diener et al. (2005) [16]. The first three clinical trial outcomes used for MA and three additional studies included in the systematic review (SR) are presented in Table 1. The primary outcome, migraine frequency (measured as attacks per month), was identified in each trial, with effect sizes calculated as Cohen’s d. The first study, Johnson et al. (1985) [14], conducted a double-blinded, placebo-controlled trial with 17 participants, where 8 received 50 mg freeze-dried feverfew powder daily for 4 months. The feverfew group showed no change in the frequency of migraine attacks, whereas the placebo group experienced an increase in frequency and severity. This suggests a strong prophylactic benefit, although conducted in a small cohort (Cohen’s d = 0.13, 95% CI: -0.82 to 1.09, p = 0.78). The second study, De Weerdt et al. (1996) [15], included 50 feverfew-naive participants who were given 0.5 mg parthenolide daily for 9 months. This trial found no statistically significant difference in migraine frequency between the feverfew and placebo groups, suggesting the absence of therapeutic effects (Cohen’s d = -0.01, 95% CI: -0.43 to 0.41, p = 0.97). The third study, Diener et al. (2005) [16] included a larger cohort of 170 participants, who received 6.25 mg of CO2-extracted feverfew (MIG-99), three times daily over 16 weeks. The migraine frequency in the treatment group was reduced by 1.9 attacks per month compared to 1.3 attacks in the placebo group, (p = 0.0456), indicating a modest but statistically significant benefit of standardized feverfew extract, (Cohen’s d = -0.39, 95% CI: -0.70 to -0.08, p = 0.01). The systematic review component additionally included three other trials that evaluated secondary migraine-related outcomes. The first, Cady et al. (2011) [17] examined a sublingual combination of feverfew and ginger, resulting in 63% of patients in the treatment group experiencing pain relief at 2 hours compared to 39% in the placebo group. Ferro et al. (2012) [18] investigated the effects of feverfew combined with acupuncture in 69 women, reporting the statistical significance (p < 0.05) of using the combination in improving quality of life and analgesic outcomes compared to acupuncture or feverfew alone. Finally, Volta et al. (2019) [19] assessed a combination of feverfew, 5-HTP and magnesium (Aurastop) in a pilot trial of 50 patients suffering migraines with aura, resulting in a 50% reduction in aura-related disability in 48 patients using Aurastop compared to 5 patients on magnesium (p < 0.001). To summarize, these studies suggest that feverfew has synergistic effects on prophylactic treatment, effective improvements in quality of life, and a reduction in the frequency of migraine attacks when paired with complementary interventions such as ginger, magnesium, or acupuncture, although further research is warranted.
Table 1: Study Designs and Outcomes of the Systematic Review Trials [14] [15] [16] [17] [18] [19].
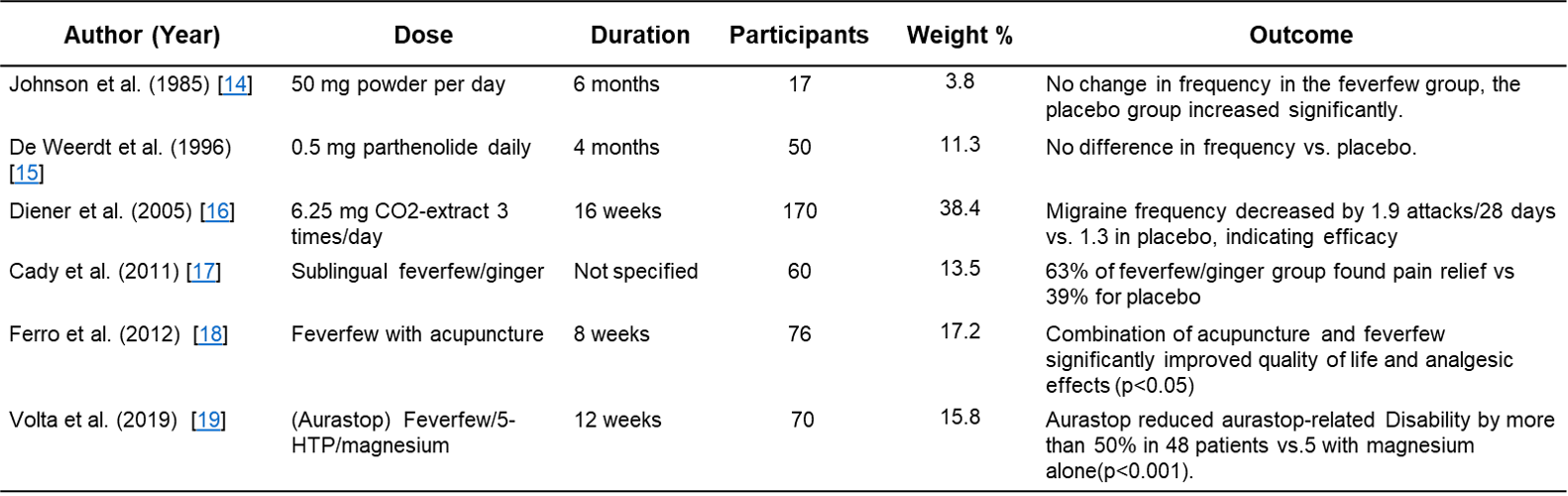
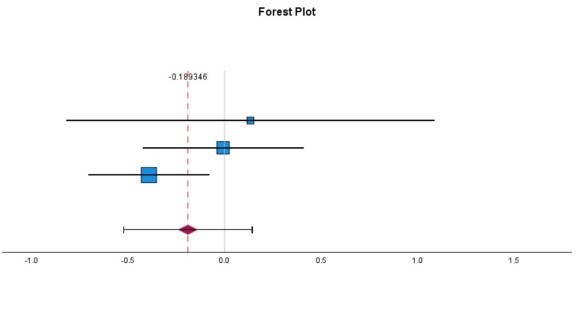
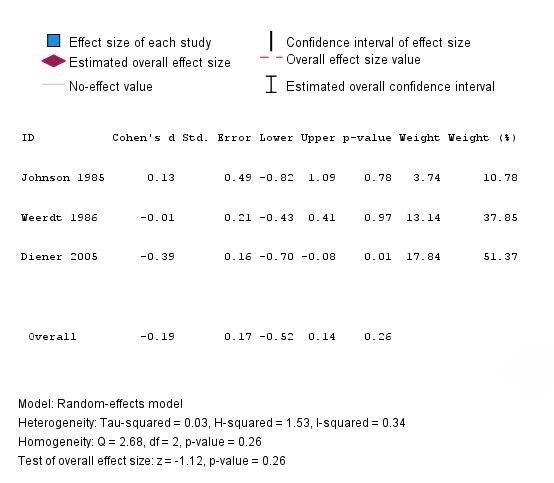
Figure 1: Forest Plot (Feverfew vs. Control): MA on Migraine Frequency (Random-Effects Model).
Limitations
This MA and systematic review are constrained by several limitations. The small sample size in the clinical trials, ranging from 17 to 170 participants, limits the statistical power to detect subtle treatment effects. Secondly, there was a variability in the formulations of feverfew used in these studies, including freeze-dried powder, parthenolide-specific formulations, and CO2-extracts. There was an inconsistency in the preparation and a lack of standardization in the key active compound, parthenolide concentration, which contributed to variable findings. The duration of interventions, which varied from 4-6 months, was insufficient to capture the long-term prophylactic and therapeutic benefits.
Conclusion
This MA of feverfew for migraine prophylaxis demonstrated a statistically insignificant outcome for the efficacy of feverfew used as a migraine prophylactic agent (Cohen’s d = –0.19, p = 0.26). However, inconsistent outcomes in terms of therapeutic effects were seen in some studies, such as using standardized CO2-extracts or combination therapies, which points to the potential benefits of using feverfew in combination therapies to obtain a synergistic benefit. Overall, larger, well-controlled trials with standardized parthenolide doses with longer follow-up are needed to determine the efficacy and therapeutic benefits of feverfew in migraine management. Future studies should also focus on larger, well-controlled trials with a standardized parthenolide content (preferably ≥ 0.2%) to provide clarity on the therapeutic roles of parthenolide.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Migraine and other headache disorders. Accessed June 26, 2025. https://www.who.int/news-room/fact-sheets/detail/headache-disorders
- Burch R, Rizzoli P, Loder E. The Prevalence and Impact of Migraine and Severe Headache in the United States: Figures and Trends From Government Health Studies. Headache. 2018;58(4):496-505. doi:10.1111/head.13281
- Costs of Migraines | Migraine Pain. Migraine Relief Center. Accessed June 30, 2025. https://www.themigrainereliefcenter.com/costs-of-migraines/
- Bonafede M, Sapra S, Shah N, Tepper S, Cappell K, Desai P. Direct and Indirect Healthcare Resource Utilization and Costs Among Migraine Patients in the United States. Headache. 2018;58(5):700-714. doi:10.1111/head.13275
- Fuakye E, Hailemeskel B, Fullas F. Migraine Management Using Feverfew, Butterbur, Peppermint, and Ginger: Perspectives of Pharmacy Students. Eur J Theor Appl Sci. 2024;2(2):412-419. doi:10.59324/ejtas.2024.2(2).35
- Tu YH, Chang CM, Yang CC, Tsai IJ, Chou YC, Yang CP. Dietary Patterns and Migraine: Insights and Impact. Nutrients. 2025;17(4):669. doi:10.3390/nu17040669
- Fibre, Magnesium And Omega 3 Fatty Acids: Food That May Help To Manage Migraine. Health and Me. Accessed June 27, 2025. https://www.healthandme.com/health-wellness/fibre-magnesium-and-omega-3-fatty-acids-food-that-may-help-to-manage-migraine-article-151115452
- Pareek A, Suthar M, Rathore GS, Bansal V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn Rev. 2011;5(9):103-110. doi:10.4103/0973-7847.79105
- Pourianezhad F, Tahmasebi S, Abdusi V, Nikfar S, Mirhoseini M. Review on feverfew, a valuable medicinal plant. J Herbmed Pharmacol. 2016;5(2):45-49.
- Jin P, Madieh S, Augsburger LL. The solution and solid state stability and excipient compatibility of parthenolide in feverfew. AAPS PharmSciTech. 2007;8(4):200. doi:10.1208/pt0804105
- Wider B, Pittler MH, Ernst E. Feverfew for preventing migraine. Cochrane Database Syst Rev. 2015;2015(4):CD002286. doi:10.1002/14651858.CD002286.pub3
- Feverfew for Prevention of Migraine | 1999-04-01 | AHC Media:…. Relias Media. Accessed June 27, 2025. https://www.reliasmedia.com/articles/53408-feverfew-for-prevention-of-migraine
- Program HF. Dietary Supplements. FDA. October 1, 2024. Accessed June 27, 2025. https://www.fda.gov/food/dietary-supplements
- Johnson ES, Kadam NP, Hylands DM, Hylands PJ. Efficacy of feverfew as prophylactic treatment of migraine. Br Med J Clin Res Ed. 1985;291(6495):569-573. doi:10.1136/bmj.291.6495.569
- De Weerdt CJ, Bootsma HPR, Hendriks H. Herbal medicines in migraine prevention: Randomized double-blind placebo-controlled crossover trial of a feverfew preparation. Phytomedicine. 1996;3(3):225-230. doi:10.1016/S0944-7113(96)80057-2
- Diener H, Pfaffenrath V, Schnitker J, Friede M, Zepelin HHH von. Efficacy and Safety of 6.25 mg t.i.d. Feverfew CO2-Extract (MIG-99) in Migraine Prevention — A Randomized, Double-Blind, Multicentre, Placebo-Controlled Study. Cephalalgia. 2005;25(11):1031-1041. doi:10.1111/j.1468-2982.2005.00950.x
- Cady RK, Goldstein J, Nett R, Mitchell R, Beach M e., Browning R. A Double-Blind Placebo-Controlled Pilot Study of Sublingual Feverfew and Ginger (LipiGesicTMM) in the Treatment of Migraine. Headache J Head Face Pain. 2011;51(7):1078-1086. doi:10.1111/j.1526-4610.2011.01910.x
- Ferro EC, Biagini AP, da Silva ÍEF, Silva ML, Silva JRT. The Combined Effect of Acupuncture and Tanacetum parthenium on quality of life in women with headache: Randomised study. Acupunct Med. 2012;30(4):252-257. doi:10.1136/acupmed-2012-010195
- Volta GD, Zavarise P, Perego L, Savi L, Pezzini A. Comparison of the Effect of Tanacethum Parthenium, 5-Hydroxy Tryptophan, and Magnesium (Aurastop) versus Magnesium Alone on Aura Phenomenon and Its Evolution. Pain Res Manag. 2019;2019(1):6320163. doi:10.1155/2019/6320163
The Effect of Feverfew on Migraine: A Meta-Analysis of Clinical Trials © 2025 by Katylin Cook, Saraf Anim, Mahmoud Mohamed Elhagaly, Randy Mullins, Mohammad Faisal Hossain is licensed under Attribution 4.0 International
Note
Place of Publication: PSciP Publishing LLC, Oakwood, VA, USA.