Abstract
According to 2021 statistics, 29.7 million people in the US and 537 million people worldwide suffer from diabetes. New therapies are constantly being investigated due to the prevalence and severity of diabetes. Often, the latest therapies come in the form of prescription medications; however, dietary supplements could also provide benefits. This Systematic Review (SR) & Meta-Analysis (MA) aims to evaluate Randomized Control Trials (RCTs) on berberine to provide an unbiased overview of its safety and efficacy in treating diabetes. Six RCTs were reviewed for the systemic review. Six articles were statistically analyzed for HbA1c (A1c) reduction, and four were analyzed for fasting plasma glucose (FPG) reduction in a meta-analysis. The results concluded that berberine reduces A1c with statistical significance as stand-alone or add-on therapy. Additionally, it did not significantly reduce FPG (p-value <0.6), but a positive trend was observed. With positive and minimal adverse effects, berberine may be beneficial in controlling diabetes.
Keywords: Berberine, Diabetes, Dietary Supplement, A Systematic Review, and Meta-Analysis
Introduction
Diabetes mellitus (DM) is a chronic disease affecting how the body turns food into energy. When food is broken down, sugar is released into the bloodstream to be used by the body for energy. To effectively use the sugar for energy, the pancreas releases insulin to remove the sugar from the blood and into the tissues. In diabetic patients, however, there is a lack of insulin being produced by the pancreas or the insulin receptors are not responding appropriately in the tissues. When this occurs, sugar remains in the blood and, if left untreated, will cause a multitude of examples throughout the body [1]. Diabetes is a significant problem in the United States and worldwide. According to 2021 statistics, 29.7 million people in the US and 537 million people worldwide suffer from diabetes [1,2]. The International Diabetes Federation predicts diabetes will affect 643 million people worldwide by 2030 and 783 million by 2045. Due to the prevalence and severity of diabetes, new therapies are sought after. These therapies are often prescribed medications, but dietary supplements could also provide benefits in the fight against diabetes.
A dietary supplement that has potential benefits for diabetes is berberine. In the human body, berberine is theorized to prevent and suppress proinflammatory cytokines, E-selectin, and genes, and increase adiponectin expression which partly explains its versatile health effects. Berberine is a nucleic acid-binding isoquinoline alkaloid with wide potential therapeutic properties [3]. Berberine is a chemical sourced from plants such as European barberry, goldenseal, goldthread, Oregon grape, phellodendron, and tree turmeric [4]. The compound is commercially available in an oral dosage form and has been used in eye drops and gels for other indications. Berberine has a pKa of 15, a logP of 3.6, an MW of 336.4 g/mol, and an oral bioavailability of 0.68% [4].
The FDA’s stance on dietary supplements is as follows: “Dietary supplements are intended to add to or supplement the diet and are different from conventional food.” Generally, It is called a drug if a product is intended to treat, diagnose, cure, or prevent diseases, even if it is labeled as a dietary supplement. Supplements are ingested and come in many forms, including tablets, capsules, soft gels, gel caps, powders, bars, gummies, and liquids” [5]. While dietary supplements are intended to treat, diagnose, cure, or prevent disease are considered “drugs,” they are not regulated in the same manner as prescription medications. Under the scope of the FDA’s Center for Food Safety and Applied Nutrition, dietary supplements are only monitored for misbranding and adulteration. Therefore, as long as dietary supplements do not include inaccurate levels or claims of their product, they are cleared by the FDA. In this case, it is the responsibility of the manufacturers and distributors to analyze their products for safety and efficacy [6,7]. The general public widely believes that dietary supplements are safe and, therefore, have no drug interactions because they are sold over the counter. This is not factual and can be harmful to individuals who believe this. Dietary supplements can cause harm in several ways, including interacting with prescription medication.[5] Due to its drug interactions, berberine should not be taken in combination with cyclosporine as it may decrease the rate at which cyclosporine is metabolized, leading to an increased risk of adverse effects. Additionally, caution should be used when combining berberine with other drugs such as dextromethorphan, losartan, CYP enzyme substrates (2C9, 2D6, 3A4), antidiabetic agents, antihypertensive agents, anticoagulants, antiplatelets, midazolam, pentobarbital, CNS depressants, and tacrolimus. Healthcare providers should also investigate if a patient is currently on any other supplements before starting berberine, as it can interact with a wide variety of dietary supplements.[5] In general, berberine does not present with a wide array of side effects. The most common side effects experienced include constipation, nausea, dyspepsia, and diarrhea. No other major adverse effects were reported from the studies [5]. The goal of this SR & MA is to evaluate the safety and efficacy data on berberine so that the readers can make informed decisions for their recommendations of the product.
Method
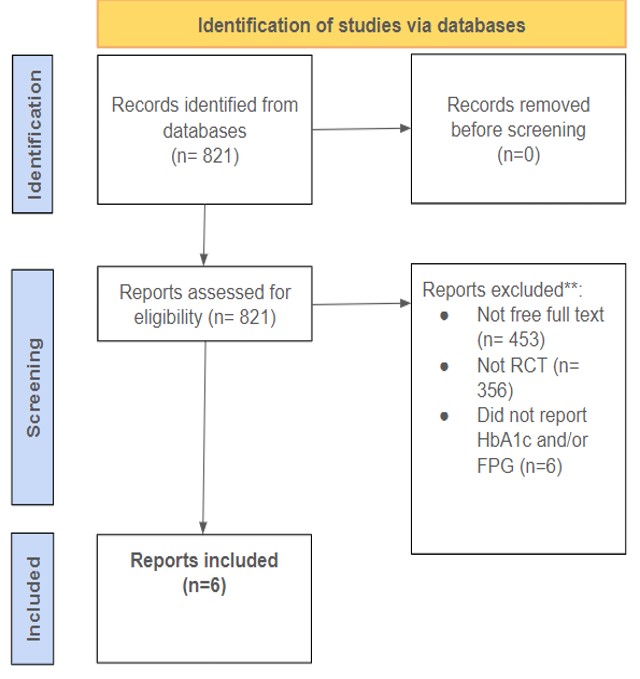
An SR & MA was performed using the electronic database PubMed. The keywords “berberine, diabetes” were used and the results were evaluated for appropriateness. To be included in this SR/MA, the study had to be available as free full text, a randomized control trial, and report A1c or FPG as an outcome measure. The search produced six articles published from 2008-2024.
Results and Discussion
Efficacy/Effectiveness:
SRs are when researchers evaluate relevant studies on a particular topic, form their conclusions, and summarize the data. MAs are more specific in the topic of discussion. MAs also differ from SRs in that researchers analyze the data statistically, typically involving heterogeneity, effect size, forest plots, and funnel plots. The purpose of heterogeneity is to determine how consistent the study is. This is evaluated with I2 – if the I2 value is >50%, the study might be inconsistent for reasons other than chance and needs to be evaluated. On the contrary, an I2 value <50% means the study is homogenous and had consistent results. “Cohen’s d is a standardized effect size for measuring the difference between two group means.” Cohen’s d is evaluated based on three categories: small (d = 0.2), medium (d = 0.5), and large (d = 0.8). These three categories refer to the effect of the treatment compared to the placebo. In this study, Cohen’s d was used to evaluate the difference between the treatment group and the control group. A forest plot is used in meta-analyses as a way to summarize the conclusions of relevant studies on a particular data point.[8]
Table 1: Study Designs and Outcomes.
| Author | Participants (#) | Study Length (weeks) | Total daily dose (mg) | Outcomes (✅/🔴) |
| Y. Zhang et al [9]. | 110 | 2 | 1000 | ✅ |
| Yin et al [10]. | 31 | 12 | 1500 | ✅ |
| H. Zhang et al [11]. | 76 | 8 | 1000 | ✅ |
| Y. Zhang et al [12]. | 201 | 12 | 1200 | ✅ |
| Sartore et al [13]. | 40 | 12 | 250 | ✅ |
| Harrison et al [14]. | 89 | 18 | 1000 & 2000 | ✅ |
Across all studies, dosing varied, with the most common total daily dose (TDD) being 1000 mg and the most common length of study being 12 weeks. Sartore et al. studied berberine as an add-on therapy to metformin at a lower daily dose, while the rest studied berberine as a stand-alone therapy. Twelve weeks was a targeted time frame for most of the studies because this is the recommended timespan to reevaluate A1c levels.
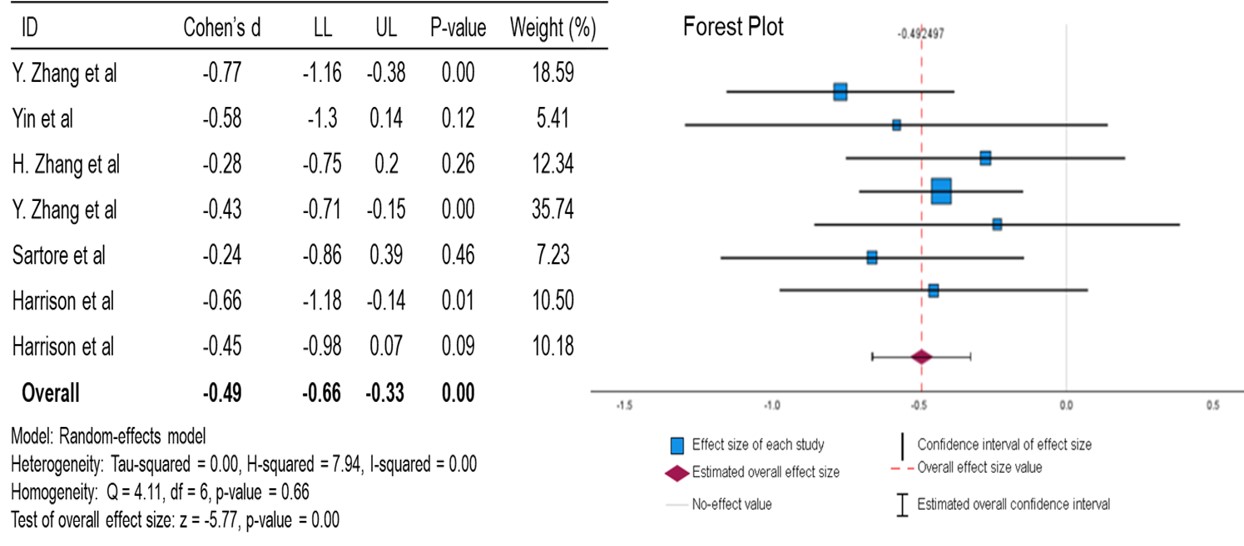
For A1c, the mean is moderately reduced compared to the null effect, with a p-value of <0.01. This data suggests a statistically significant moderate effect of berberine in lowering the A1c of diabetic patients. An I2 value of 0.00 (zero to low heterogeneity) and an overall Cohen’s d of -0.49 confirm that berberine exhibits a moderate effect on lowering A1c in diabetic patients.
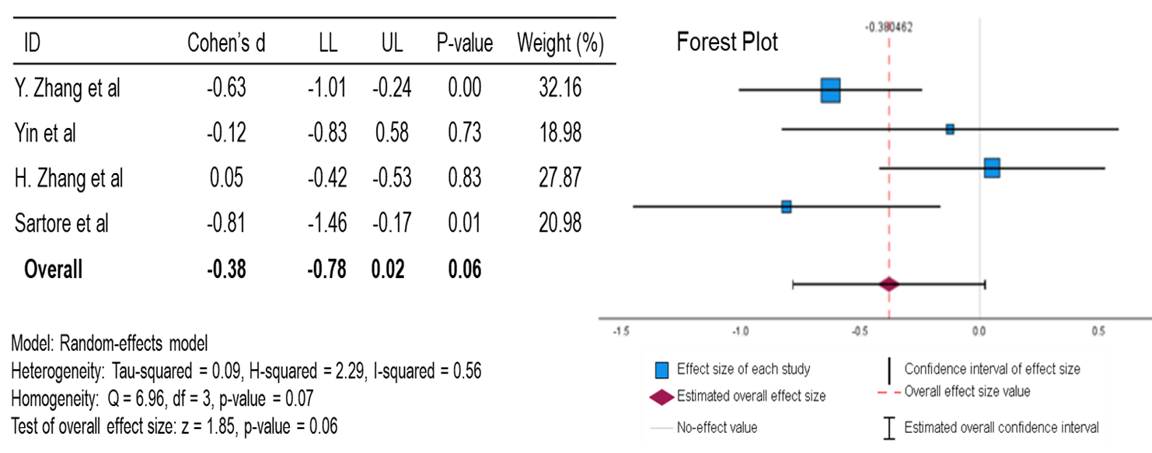
For FPG, the mean is slightly to moderately reduced compared to the null effect, with a p-value of 0.06. This data suggests a positive correlation between berberine and lowering FPG, but this is not statistically significant. With an I2 of 0.56 (moderate heterogeneity), there is potential for inconsistency in the results that are not attributable to random chance. However, with a Cohen’s d of -0.38, a positive correlation exists between berberine and lowering FPG.
While the results for FPG were not statistically significant, combined with the statistically significant A1c results, berberine supplementation may provide benefits in diabetic patients. Therefore, it is within reason to consider berberine for diabetic patients struggling to lower A1c and FPG on their current antidiabetic regimens.
Conclusion
Berberine shows excellent potential in the supplementation of antidiabetic regimens. After reviewing the randomized control trials used for this SR & MA, the positive trends in FPG and the statistically significant reduction in A1c (overall Cohen’s d of -0.49) support berberine’s use in type 2 diabetic patients. Before starting berberine patients should consult a healthcare provider. Exclusively reviewing randomized control trials for this SR & MA enhanced the validity of the findings. In 5 of these articles, berberine was studied as a stand-alone therapy, but it may provide better benefits if combined, at a lower dose, with other antidiabetic agents. Berberine could also provide benefits in preventing diabetes for pre-diabetic patients as an adjunct therapy to lifestyle changes. With the positive outcomes of the included articles, berberine’s antidiabetic properties are to be seriously considered for future diabetic research mentioned above.
Conflict of interest
The authors declare no conflict of interest.
References
- National Diabetes Statistics Report | Diabetes | CDC. Published November 29, 2023. Accessed March 11, 2024.
- Home, Resources, diabetes L with, et al. IDF Diabetes Atlas. Accessed March 11, 2024.
- Berberine: MedlinePlus Supplements. Accessed March 11, 2024.
- PubChem. Berberine. Accessed March 11, 2024.
- BERBERINE: Overview, Uses, Side Effects, Precautions, Interactions, Dosing and Reviews. Accessed March 11, 2024.
- Commissioner O of the. FDA 101: Dietary Supplements. FDA. Published online June 9, 2022. Accessed March 11, 2024.
- Nutrition C for FS and A. Dietary Supplements. FDA. Published February 16, 2024. Accessed March 11, 2024.
- Harris J, Webb M, Hossain MF. Evaluating the Efficacy of Glucosamine and Chondroitin for the Supplemental Treatment of Osteoarthritis: A Systematic Review and Meta-Analysis. Am J Nat Med Facts. 2024;1(1):1-6.
- Zhang Y, Li X, Zou D, et al. Treatment of Type 2 Diabetes and Dyslipidemia with the Natural Plant Alkaloid Berberine. J Clin Endocrinol Metab. 2008;93(7):2559-2565. doi:10.1210/jc.2007-2404
- Yin J, Xing H, Ye J. Efficacy of Berberine in Patients with Type 2 Diabetes. Metabolism. 2008;57(5):712-717. doi:10.1016/j.metabol.2008.01.013
- Zhang H, Wei J, Xue R, et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59(2):285-292. doi:10.1016/j.metabol.2009.07.029
- Zhang Y, Gu Y, Ren H, et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020;11:5015. doi:10.1038/s41467-020-18414-8
- Sartore G, Ragazzi E, Antonello G, Cosma C, Lapolla A. Effect of a New Formulation of Nutraceuticals as an Add-On to Metformin Monotherapy for Patients with Type 2 Diabetes and Suboptimal Glycemic Control: A Randomized Controlled Trial. Nutrients. 2021;13(7):2373. doi:10.3390/nu13072373
- Harrison SA, Gunn N, Neff GW, et al. A phase 2, proof of concept, randomized controlled trial of berberine ursodeoxycholate in patients with presumed non-alcoholic steatohepatitis and type 2 diabetes. Nat Commun. 2021;12:5503. doi:10.1038/s41467-021-25701-5
Berberine, Nature’s Answer to Diabetes Management: A Systematic Review and Meta-Analysis © 2024 by McKinley Webb, Janessa Harris, Mohammad Faisal Hossain is licensed under Attribution 4.0 International
Note
Place of Publication: PSciP Publishing LLC, Oakwood, VA, USA.